How Many Neutrons in Nitrogen 15
Nitrogen 15 has an atomic mass of 15. The number of neutrons varies depending on the isotope.

Notation For Isotopes Of Nitrogen N Youtube
So for the element of NITROGEN you already know that the atomic number tells you the number of electrons.
. How many protons neutrons and electrons does nitrogen 20 have. This problem has been solved. That means 6 protons.
Therefore Nitrogen-14 has 7 protons 7 neutrons and 7 electrons. Therefore the number of protons neutrons and electrons are 7 8 and 7 respectively. N-15 has 7 electrons because it has 7 protons and p e.
The mass number is protons plus of neutrons so for N-15 mass is 15 and the protons are always 7 so there have to be 15-78 neutrons. Hence seven protons eight neutrons and seven electrons make up an atom of nitrogen-15. The mass number is protons plus of neutrons so for N-15 mass is 15 and the protons are always 7 so there have to be 15-78 neutrons.
Each atom of nitrogen-15 contains 7 protons 8 neutrons and 7 electrons. Expert Answer 100 2 ratings Nitrogen atomic number is 7. Since the two isotopes have different amount of neutrons they will have different masses and we conclude that their mass numbers are different from each other.
Hence seven protons eight neutrons and seven electrons make up an atom of nitrogen-15. 3 rows Nitrogen 15 has an atomic mass of 15. The isotope nitrogen-15 has 7 protons and electrons and 8 neutrons.
View the full answer Previous question Next question. How many neutrons are present in nitrogen. 1 on a question The atomic number of nitrogen is 7.
The number of protons determines physical identity of all elements. Above it says Carbon-12. The mass number is protons plus of neutrons so for N-15 mass is 15 and the protons are always 7 so there have to be 15-7 8 neutrons.
Nitrogen 15 has an atomic mass of 15. 3 rows Nitrogen-15 is composed of 7 protons 8 neutrons and 7 electrons. Nitrogen-15 would have 8 neutrons nitrogen-16 would have 9 neutrons and so on.
Nitrogen 15 has an atomic mass of 15. The mass number is protons plus of neutrons so for N- 15 mass is 15 and the protons are always 7 so there have to be 15 -78 neutrons. See the answer how many neutrons are contained in nitrogen -15.
An easy way to find the number of neutrons in an atom would be to look at the atomic mass in amu and subtract the number of protons from it. All nitrogen atoms by definition have 7 protons. So the number of neutrons in the nitrogen atom is eight.
Nitrogen atoms all have 7 protons. Mass number - Atomic number neutrons 12-6 6 neutrons. Nitrogen 15 has an atomic mass of 15.
That number of protons identifies it as nitrogen. 15 - 7 8. So the number of neutrons in the nitrogen atom is eight.
Nitrogen has an atomic number of 7 Z7 because it has 7 protons in its nucleus. Answer 1 of 5. Some nitrogen atoms have an atomic mass number of 15 A15.
In this case of Nitrogen isotope where the mass number is 15 the number of neutron will be mass number - number of protons ie. A number of proton number of neutron. Nitrogen has 7 protons an.
Does nitrogen have 7 electrons. The mass number is protons plus of neutrons so for N-15 mass is 15 and the protons are always 7 so there have to be 15- 7 8 neutrons. Nitrogen 15 has an atomic mass of 15.
N- 15 has 7 electrons because it has 7 protons and p e. Once that is set the number of. N-15 has 7 electrons because it has 7 protons and p e.
Do all nitrogen have 7 protons. Nothing really changes in their atomic structure. Nitrogen-20 Nitrogen is atomic number 7.
How many protons and neutrons does nitrogen 16 have. The mass number denoted by A is the sum of the number of protons and neutrons in an atom ie. 20-7 13 This isotope of nitrogen has 13 neutrons.
Two sources of. So it has 7 protons. How many protons neutrons and electrons make up an atom of nitrogen-15.
One neutral atom of nitrogen has seven protons seven neutrons and seven electrons. This element is found in group 15 and period 2 of the Periodic Table of the Elements. 6 protons 6 neutronsand so on.
N-15 has 7 electrons because it has 7 protons and p e. N-15 has 7 electrons because it has 7 protons and p e. That means there are 7 electrons in a nitrogen atom.
Nitrogen-15Nitrogen-20 of protons77 of neutrons813 of electrons77. Isotopes vary only in the number of neutrons present. How many protons neutrons and electrons are in an atom of nitrogen - 15.
12 is the mass number. The mass number is protons plus of neutrons so. They will have different amount of neutrons there are 7 neutrons in 14N and 8 neutrons in 15N.
It has an atomic weight of 14007 amu.

How To Find The Number Of Protons Electrons Neutrons For Nitrogen N Youtube
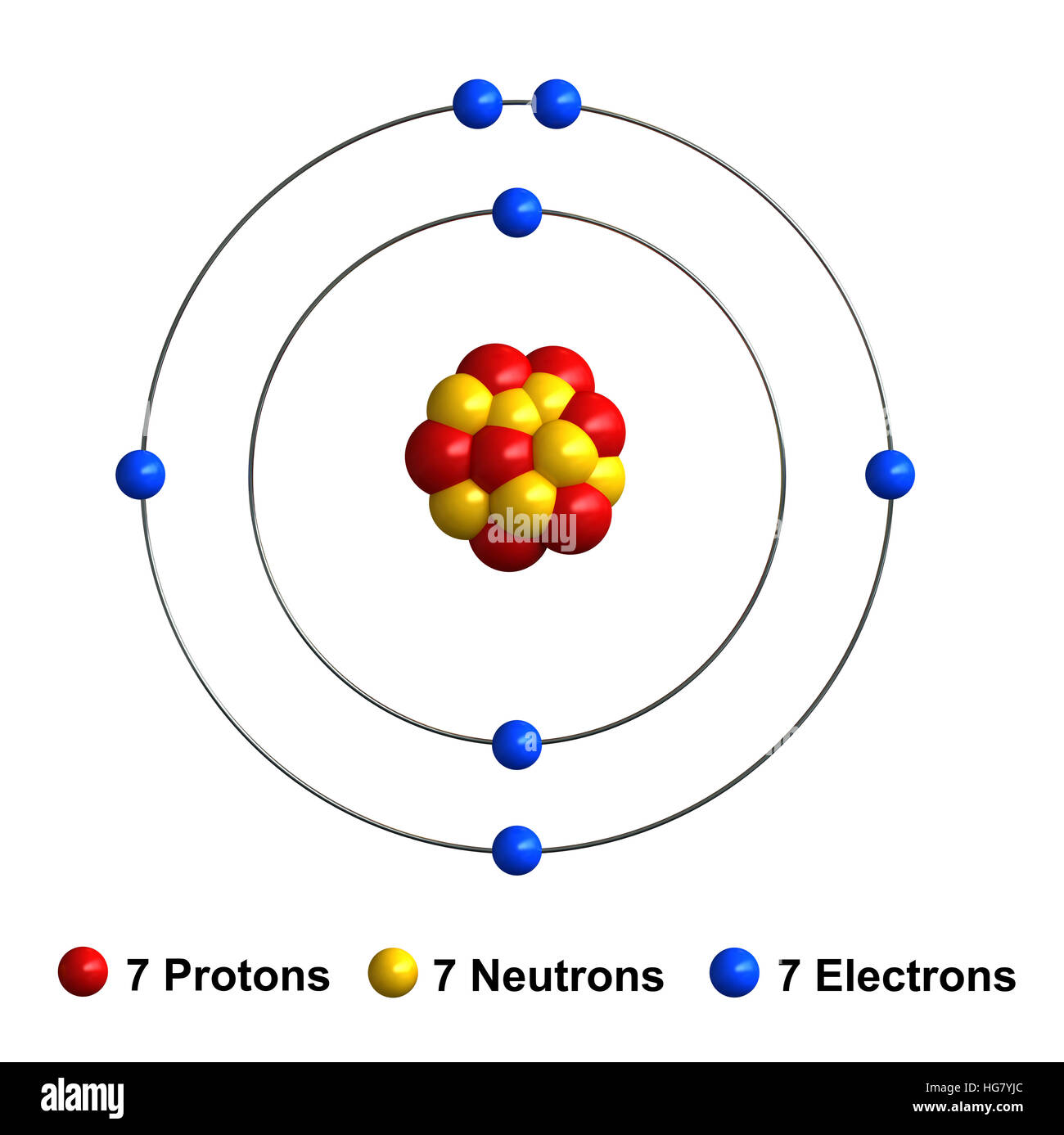
Nitrogen Atom High Resolution Stock Photography And Images Alamy

Solved How Many Protons Neutrons And Electrons Are There In Chegg Com
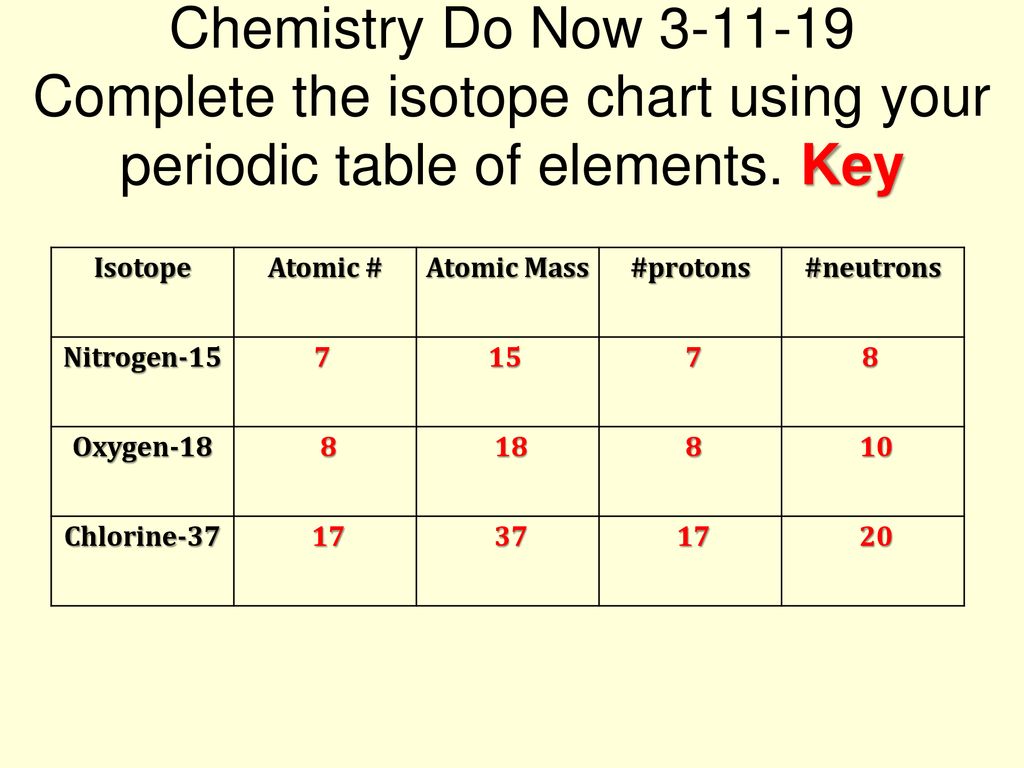
Atomic Atomic Mass Protons Neutrons Nitrogen 15 Oxygen Ppt Download

Isotopes And Ions Fill In The Following Table Symbol Atomic Mass Atomic Number Of Protons Of Neutrons Of Electrons Na Na Ne Ne Hg Hg Zn Zn Al Ppt Download
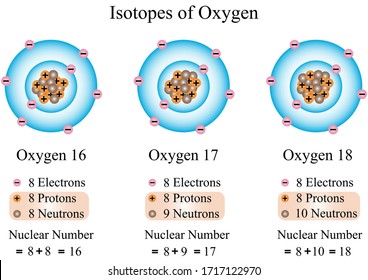
Illustration Chemical Isotopes Nitrogen All Atoms Stock Vector Royalty Free 1717122958
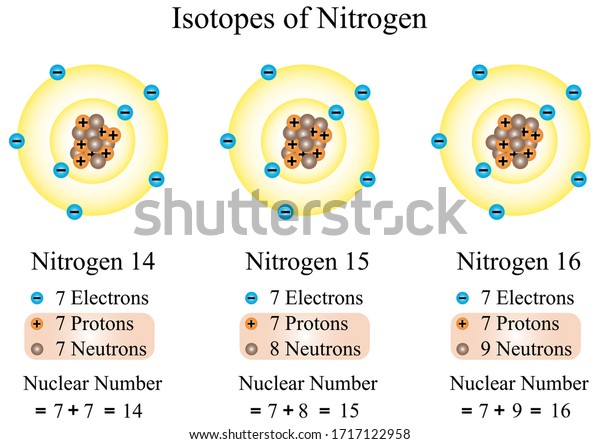
Illustration Chemical Isotopes Nitrogen All Atoms Stock Vector Royalty Free 1717122958

What Is The Mass Number Of Nitrogen Lisbdnet Com

How Many Protons Does A Nitrogen Atom Have Lisbdnet Com
How Many Protons Does Nitrogen Always Have Quora
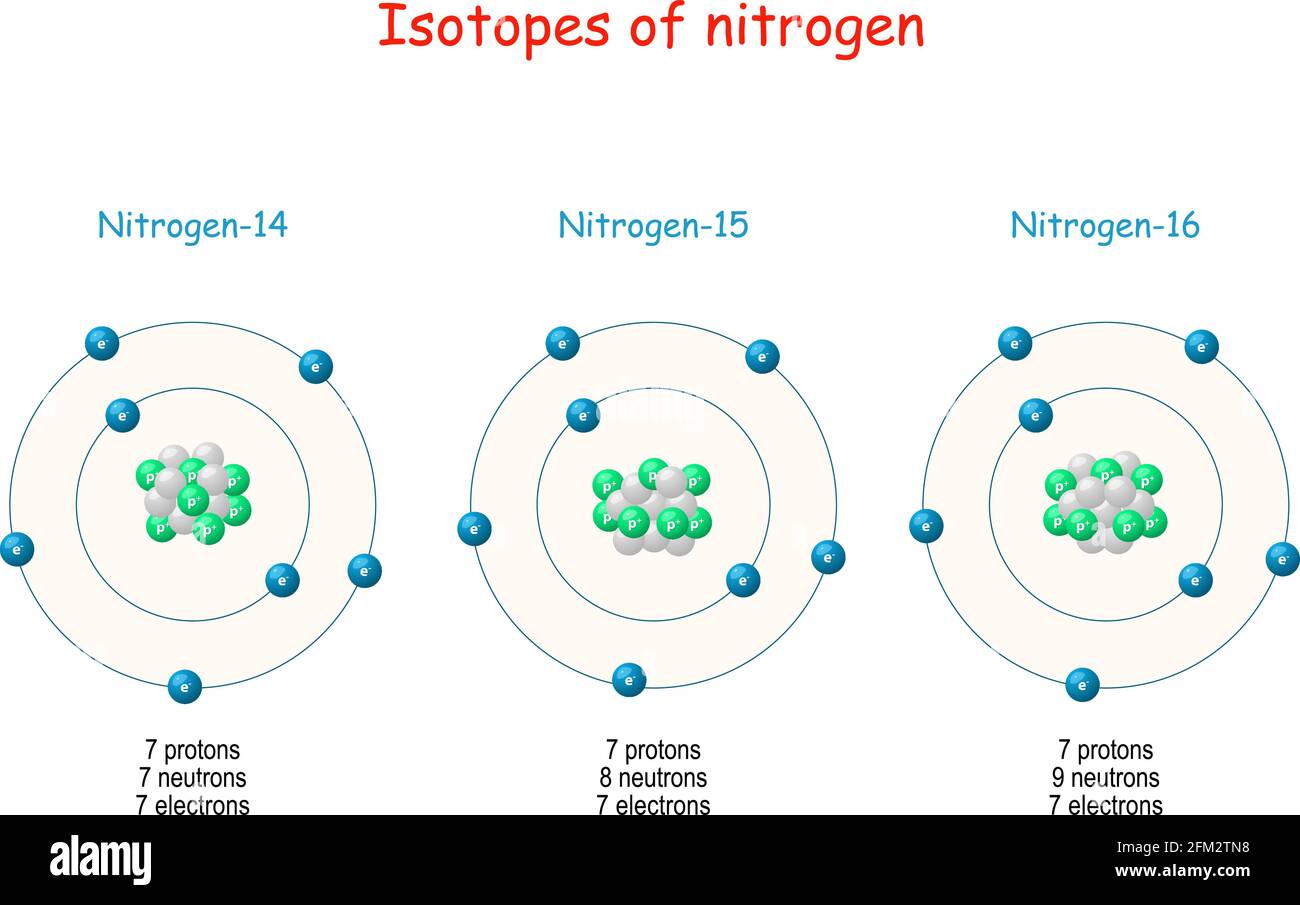
Nitrogen Isotopes Structure Of Atom Labeled Scheme With Particles Protons Neutrons And Electrons Vector Illustration For Science Education Stock Vector Image Art Alamy
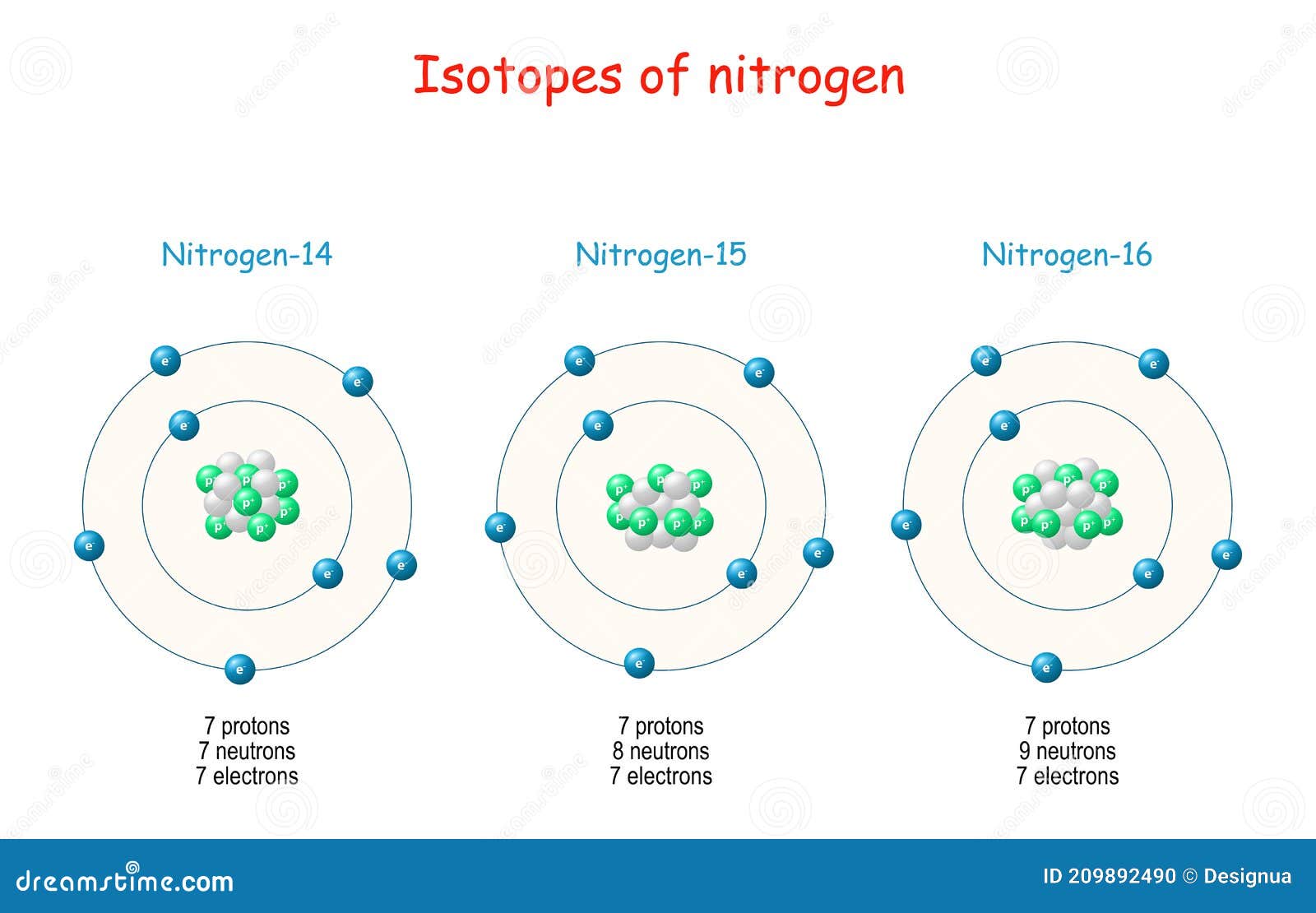
Nitrogen Isotopes Structure Of Atome Stock Vector Illustration Of Micro Neutron 209892490

Nitrogen Protons Neutrons Electrons Electron Configuration
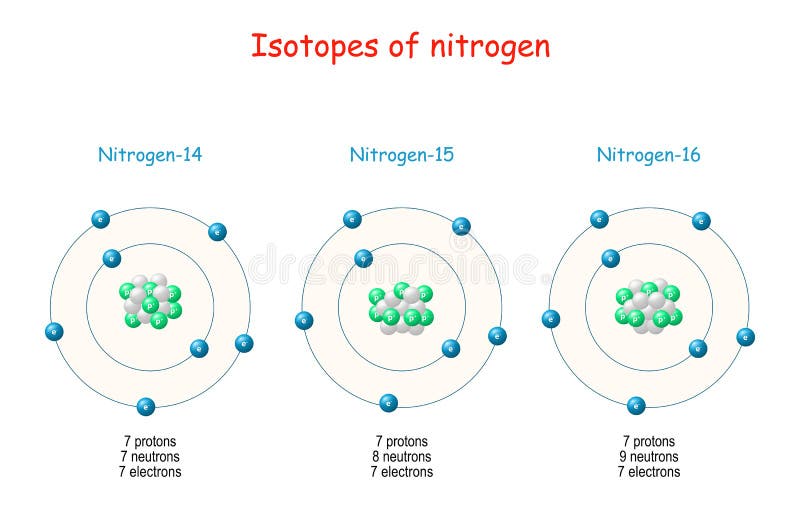
Nitrogen Isotopes Structure Of Atome Stock Vector Illustration Of Micro Neutron 209892490

How Many Electrons Does An Isotope Of Nitrogen 15 Always Have Quora

Nitrogen Protons Neutrons Electrons Electron Configuration
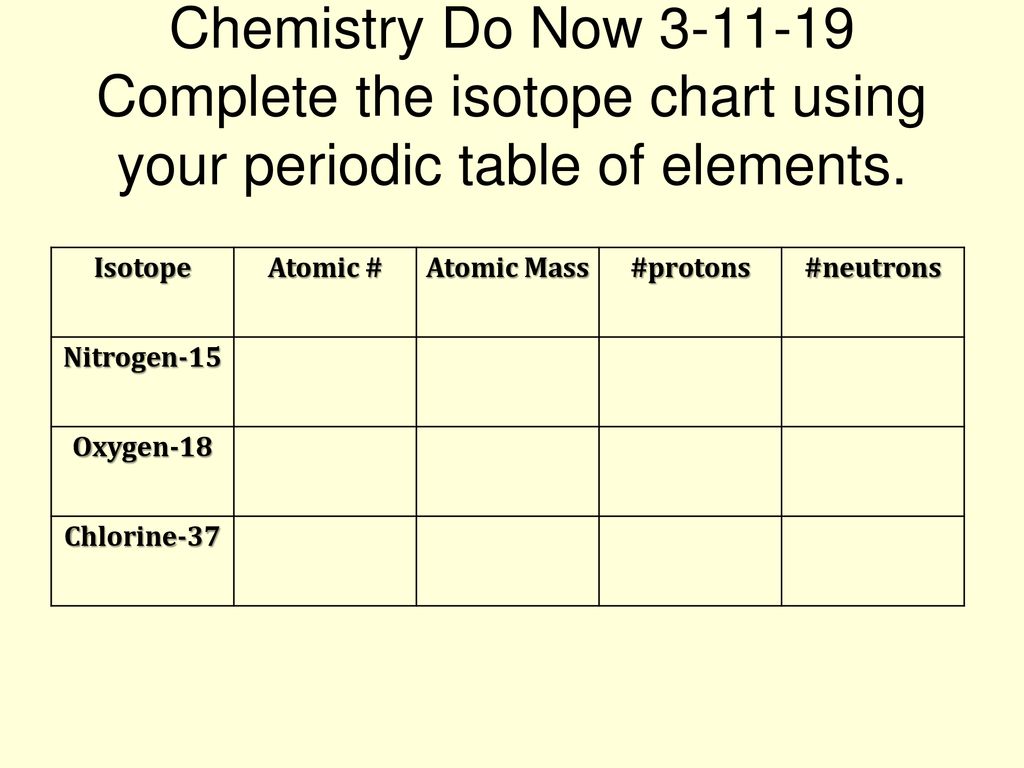
Atomic Atomic Mass Protons Neutrons Nitrogen 15 Oxygen Ppt Download
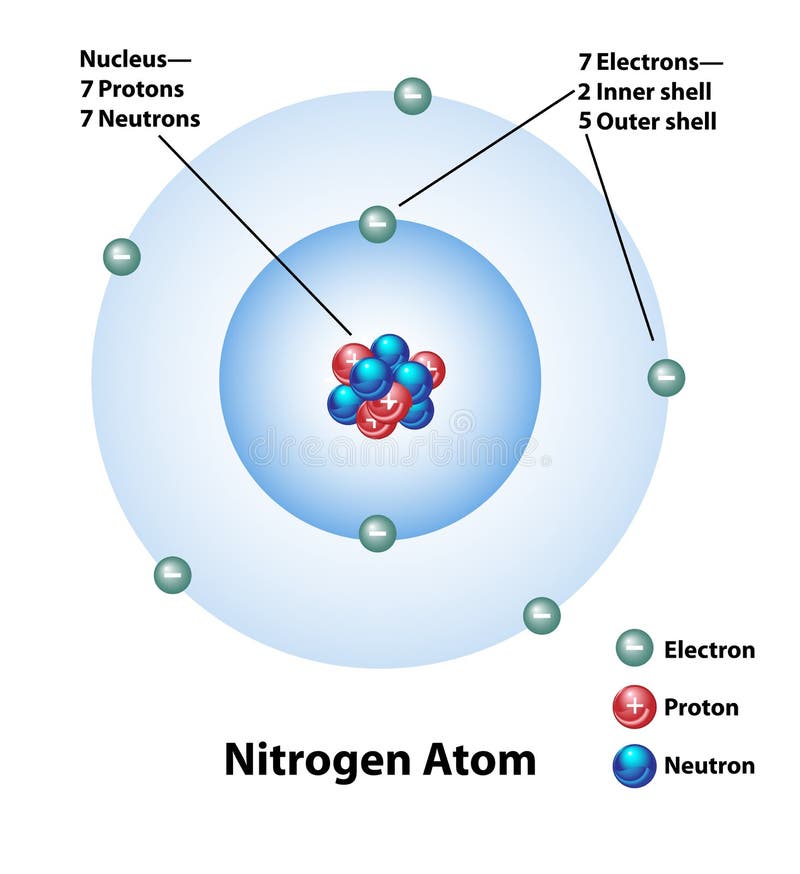
Nitrogen Atom Diagram Stock Illustrations 64 Nitrogen Atom Diagram Stock Illustrations Vectors Clipart Dreamstime

Comments
Post a Comment